16 Apr 2020

Research Officer in CHeBA’s Genetics & Epigenomics Group, Naga Mutyala, completed her PhD in March 2020 which focused on evaluating a rodent model and novel treatments for glioblastoma, a grade IV brain cancer, using gene expression profiling.
Glioblastoma is the most lethal grade of brain cancer in adults and resultantly has very poor patient outcomes with a median survival rate of only 15 months.
To improve treatment success further evaluation of alternative therapies is urgently required. Therefore, we examined the efficacy of an antipsychotic drug olanzapine as an alternative treatment to combat this insidious disease.
Research has indicated that olanzapine is effective in reducing cancer incidence in schizophrenia patients (Catts et al., 2008 & Mortensen, 1994). The drug presents as an attractive alternative treatment as it has already been approved in terms of safety for human consumption and dosage limits. Thus, considerably reducing the time and costs required to implement drug treatment which is of great importance in glioblastoma management.
An analysis of human glioblastoma at a molecular level revealed four subtypes, classical, mesenchymal, neural and proneural which each have differing treatment efficacy and prognosis.
So far animal models resembling specific molecular subtypes of glioblastoma have not been not identified (Lenting et al., 2017). In this thesis, we studied F98 rat brain tumour model and additionally, using the same animal model of the disease we also evaluated the effect of olanzapine, an antipsychotic drug alone and in combination with a chemotherapeutic temozolomide; a standard drug used to treat glioblastoma.
Through surgically implanting tumour cells in rats the disease was mimicked and then treated with olanzapine, temozolomide and a combination therapy. Following treatment tumour growth (Figure 1) and animal survival were recorded (Figure 2).
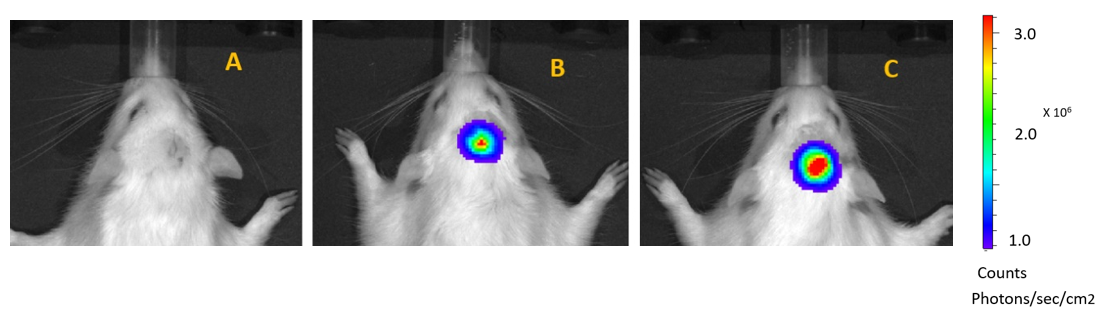
Figure 1: Bioluminescence imaging of tumours in rats. A: No bioluminescence– no tumour, B: low bioluminescence – small tumour, C: high bioluminescence – large tumour.
Experimental results suggest the combination of chemotherapeutic and antipsychotic drug treatment improved longevity in rats by suppressing expression of tumour promoting genes. The rat brain model studied most closely identified with the mesenchymal disease subtype found in humans and can therefore be used in future to develop alternative treatments for this subtype of disease.
The implementation of alternative therapies to manage glioblastoma may be key to better disease treatment.
Tailoring treatment to specific disease subtypes is more effective than standardised therapy, and this has significant implications for current models of patient care and treatment outcomes.
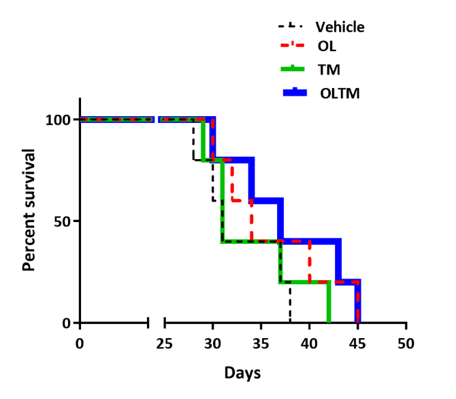
Figure 2: Kaplan Meier survival curve for F98 glioblastoma rats (n=20). Animals treated with olanzapine and temozolomide in combination showed a modest survival advantage over those treated with either drug alone. Each treatment group included five rats.
Naga Mutyala works alongside CHeBA’s Genetics & Epigenomics Group Leader, Dr Karen Mather, with a research focus on gaining better understanding of the genetic and epigenetic factors involved in ageing and age-related disorders. Ms Mutyala’s PhD was supervised by Associate Professor Louise Lutze-Mann and CHeBA’s Older Australian Twins Study Coordinator, Dr Vibeke Catts.
